Does immune enhancement of disease occur in human coronavirus infections?
There are seven CoV serotypes associated with disease in humans: four that cause the common cold (OC43, NL63, 229E, and HKU1) and three that are highly pathogenic (SARS-CoV-1, SARS-CoV-2, and MERS-CoV).
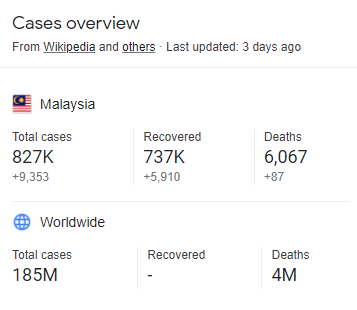
Ninety percent of adults are seropositive for coronavirus strains causing the common cold .
A clinical study where participants were experimentally infected twice, 1 year apart, with CoV 229E did not report enhanced disease; after the second exposure, the time during which virus was shed in nasal secretions was reduced, and there were no symptoms of disease.
Both serum and nasal IgA antibodies specific for CoV 229E were associated with a decreased period of nasal virus shedding. Immune enhancement of SARS-CoV-2 infection attributable to cross-reactive common cold CoV antibodies has not been reported so far.
Rather, prior infection with common cold CoVs has been suggested to be either potentially protective by virtue of inducing antibodies that cross-react with the SARS-CoV-2 spike protein subunit S2 (68) or to be the source of SARS-CoV-2–reactive neutralizing antibodies that arose in a patient with SARS-CoV-1 who recovered from SARS-CoV-1 infection.
Regarding T cell immunity to common cold CoVs, ~40 to 60% of individuals who have not been exposed to SARS-CoV-2 have SARS-CoV-2–reactive CD4+ T cells, suggesting that there is cross-reactive T cell recognition between common cold CoVs and SARS-CoV-2.
So far, there is no direct evidence suggesting that preexisting immunity to common cold CoVs is detrimental to the outcome of SARS-CoV-2 infection.
Reports correlating antibody responses and disease severity are conflicting and confounded by higher viral loads and the potential for more immune stimulation with severe SARS-CoV-2 infection.
Studies of MERS-CoV have shown increased neutralizing antibodies or an increased duration of spike protein–binding antibody in severe disease.
Among 128 SARS-CoV-1–infected individuals, the amount of neutralizing antibodies was not associated with disease severity.
However, one report suggested that increased antibody production correlated with increased respiratory failure in humans infected with SARS-CoV-1.
In contrast, another study showed no difference in time to seroconversion in SARS-CoV-1–infected individuals who survived compared with those who died.
The presence of SARS-CoV-1–specific IgG 10 days after onset of symptoms was associated with a decrease in nasopharyngeal viral load and with worsening of clinical disease in ~20% of individuals with respiratory failure requiring ventilator support.
Use of a pseudovirus and a plaque reduction neutralization test (PRNT) assay to study acutely ill and recovered SARS-CoV-1–infected patients showed a decrease in viral load coincident with the time of seroconversion, suggesting that the neutralizing antibody response may play a role in clearance of virus.
In the setting of SARS-CoV-1 infection, it has been reported that CD4+ T cell responses correlated with positive outcomes in mice, but more severe disease in humans.
Tan et al. have suggested that IgM and IgG against the SARS-CoV-2 nucleocapsid protein increased in patients with severe compared with mild COVID-19 disease.
Systems analysis of serological signatures in COVID-19 disease revealed that functional antibody responses to SARS-CoV-2 nucleocapsid protein were elevated in those who died, whereas spike-specific antibody responses were enriched among convalescent individuals.
A clinical study of 175 patients with COVID-19 reported that higher serum neutralizing antibody titers may be associated with lower lymphocyte counts and higher C-reactive protein, but the amount of neutralizing antibodies in severe compared with mild disease was not reported.
Studies have reported higher SARS-CoV-2 neutralizing antibody titers in old compared with young patients with COVID-19. One study reported higher IgM and IgG antibodies against SARS-CoV-2 spike and nucleocapsid proteins in patients with severe compared with mild COVID-19 disease.